| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:44:05 UTC |
|---|
| Update Date | 2020-05-21 16:28:55 UTC |
|---|
| BMDB ID | BMDB0001319 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Pyridoxine 5'-phosphate |
|---|
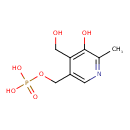
| Description | Pyridoxine 5'-phosphate, also known as pyridoxine 5'-phosphate or Pyridoxine 5'-phosphate, belongs to the class of organic compounds known as pyridoxine 5'-phosphates. These are pyridoxines that carry a phosphate group at the 5-position. Pyridoxine 5'-phosphate is possibly soluble (in water) and a very strong basic compound (based on its pKa). Pyridoxine 5'-phosphate exists in all living species, ranging from bacteria to humans. Pyridoxine 5'-phosphate participates in a number of enzymatic reactions, within cattle. In particular, Pyridoxine 5'-phosphate can be biosynthesized from pyridoxine; which is mediated by the enzyme pyridoxal kinase. In addition, Pyridoxine 5'-phosphate can be converted into pyridoxal 5'-phosphate; which is catalyzed by the enzyme pyridoxine 5'-phosphate oxidase. In cattle, pyridoxine 5'-phosphate is involved in the metabolic pathway called the vitamin B6 metabolism pathway. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Pyridoxine 5'-(dihydrogen phosphate) | ChEBI | | Pyridoxine 5-phosphate | ChEBI | | Pyridoxine phosphate | ChEBI | | PYRIDOXINE-5'-phosphATE | ChEBI | | Pyridoxol 5'-phosphate | ChEBI | | Pyridoxol 5-phosphate | ChEBI | | Pyridoxyl-5-phosphate | ChEBI | | Pyridoxine 5'-(dihydrogen phosphoric acid) | Generator | | Pyridoxine 5-phosphoric acid | Generator | | Pyridoxine phosphoric acid | Generator | | PYRIDOXINE-5'-phosphoric acid | Generator | | Pyridoxol 5'-phosphoric acid | Generator | | Pyridoxol 5-phosphoric acid | Generator | | Pyridoxyl-5-phosphoric acid | Generator | | Pyridoxine 5'-phosphoric acid | Generator | | 5-Hydroxy-4-(hydroxymethyl)-6-methyl-3-pyridylmethyl dihydrogen phosphate | HMDB | | 5-Hydroxy-6-methyl-3,4-pyridinedimethanol alpha( 3)-(dihydrogen phosphate) | HMDB | | Pyridoxine-5P | HMDB | | Pyridoxine-p | HMDB | | Pyridoxine-phosphate | HMDB | | Pyridoxine 5-phosphate hydrochloride | HMDB | | 5-Phosphopyridoxine | HMDB |
|
|---|
| Chemical Formula | C8H12NO6P |
|---|
| Average Molecular Weight | 249.1577 |
|---|
| Monoisotopic Molecular Weight | 249.040223633 |
|---|
| IUPAC Name | {[5-hydroxy-4-(hydroxymethyl)-6-methylpyridin-3-yl]methoxy}phosphonic acid |
|---|
| Traditional Name | pyridoxine-5'-phosphate |
|---|
| CAS Registry Number | 447-05-2 |
|---|
| SMILES | CC1=NC=C(COP(O)(O)=O)C(CO)=C1O |
|---|
| InChI Identifier | InChI=1S/C8H12NO6P/c1-5-8(11)7(3-10)6(2-9-5)4-15-16(12,13)14/h2,10-11H,3-4H2,1H3,(H2,12,13,14) |
|---|
| InChI Key | WHOMFKWHIQZTHY-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyridoxine-5'-phosphates. These are pyridoxines that carry a phosphate group at the 5-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pyridines and derivatives |
|---|
| Sub Class | Pyridoxines |
|---|
| Direct Parent | Pyridoxine-5'-phosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyridoxine-5'-phosphate
- Monoalkyl phosphate
- Hydroxypyridine
- Methylpyridine
- Organic phosphoric acid derivative
- Alkyl phosphate
- Phosphoric acid ester
- Heteroaromatic compound
- Azacycle
- Hydrocarbon derivative
- Alcohol
- Aromatic alcohol
- Organic nitrogen compound
- Primary alcohol
- Organooxygen compound
- Organonitrogen compound
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0002-9530000000-1e967afad92165e82bee | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00di-9226000000-49f5293644ca681d401b | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0ue9-0590000000-68640055ed308d852fd0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f89-0900000000-1450b9f084acf6421d59 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-4900000000-8fd6492e6f2a6bea9879 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-8090000000-65f73f2c7a460a3fca23 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-9010000000-5b0859a6a40a458fcc9f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-a6fead236e12d88986b8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0290000000-da88c1789911b79c0362 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ff0-0910000000-366dc9237952c888f4b8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-2900000000-cd37cb30cd544c5f866a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-002b-9010000000-64a9d47104102ec4486f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-9000000000-ef724d5177083af378f0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-a5e502a2627af2048a1f | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|