| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:44:27 UTC |
|---|
| Update Date | 2020-05-21 16:27:02 UTC |
|---|
| BMDB ID | BMDB0001343 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
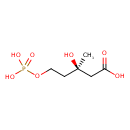
| Common Name | Mevalonic acid-5P |
|---|
| Description | Mevalonic acid-5P, also known as mevalonate-5P or 5-phosphomevalonate, belongs to the class of organic compounds known as monoalkyl phosphates. These are organic compounds containing a phosphate group that is linked to exactly one alkyl chain. Mevalonic acid-5P is a very hydrophobic molecule, practically insoluble (in water), and relatively neutral. Mevalonic acid-5P exists in all living species, ranging from bacteria to humans. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (R)-5-Phosphomevalonate | ChEBI | | (R)-5-Phosphomevaloonic acid | ChEBI | | (R)-Mevalonic acid 5-phosphate | ChEBI | | (R)-5-Phosphomevalonic acid | Generator | | (R)-5-Phosphomevaloonate | Generator | | (R)-Mevalonate 5-phosphate | Generator | | (R)-Mevalonic acid 5-phosphoric acid | Generator | | Mevalonate-5P | Generator | | 5-Phosphomevalonate | HMDB | | Mevalonate-5-p | HMDB | | Mevalonate-5-phosphate | HMDB | | Mevalonate-p | HMDB | | p-Mevalonate | HMDB | | 5-Phosphomevalonic acid | HMDB | | Mevalonate 5-phosphate | HMDB | | Phosphomevalonate | HMDB | | Phosphomevalonic acid | HMDB | | Phosphomevalonic acid, (+-)-isomer | HMDB | | (3R)-3-Hydroxy-3-methyl-5-(phosphonooxy)pentanoic acid | HMDB | | 3-Hydroxy-3-methyl-5-(phosphonooxy)pentanoic acid | HMDB | | Mevalonic acid phosphate | HMDB | | Mevalonic acid-5P | HMDB |
|
|---|
| Chemical Formula | C6H13O7P |
|---|
| Average Molecular Weight | 228.137 |
|---|
| Monoisotopic Molecular Weight | 228.039889282 |
|---|
| IUPAC Name | (3R)-3-hydroxy-3-methyl-5-(phosphonooxy)pentanoic acid |
|---|
| Traditional Name | mevalonate-5-phosphate |
|---|
| CAS Registry Number | 73566-35-5 |
|---|
| SMILES | C[C@@](O)(CCOP(O)(O)=O)CC(O)=O |
|---|
| InChI Identifier | InChI=1S/C6H13O7P/c1-6(9,4-5(7)8)2-3-13-14(10,11)12/h9H,2-4H2,1H3,(H,7,8)(H2,10,11,12)/t6-/m1/s1 |
|---|
| InChI Key | OKZYCXHTTZZYSK-ZCFIWIBFSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as monoalkyl phosphates. These are organic compounds containing a phosphate group that is linked to exactly one alkyl chain. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Organic phosphoric acids and derivatives |
|---|
| Sub Class | Phosphate esters |
|---|
| Direct Parent | Monoalkyl phosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Monoalkyl phosphate
- Short-chain hydroxy acid
- Fatty acid
- Tertiary alcohol
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0292-9600000000-a4f04a66624afb8d522a | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00di-9413000000-7329637530b2ddc29e0b | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-1960000000-a02358583ea07452bacf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03e9-4900000000-ef07c3371552f7c1c11d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-02ti-9800000000-bba2c1c56648b04a1fdd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-8960000000-de18bb77f2282c89d45a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-9100000000-6dd83d59bdb427eeee24 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-9c715a4954dce4d5a856 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01si-1940000000-c405e8487510c01d67be | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0229-9300000000-84bcd8640d320dd874e3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-06ry-9200000000-6589ef1abdd604511639 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-5090000000-98125cefd75fccd3e564 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-9020000000-74f440cb4e3ffa40388d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-a5e502a2627af2048a1f | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|