| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:45:37 UTC |
|---|
| Update Date | 2020-05-21 16:28:34 UTC |
|---|
| BMDB ID | BMDB0001416 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Pantetheine 4'-phosphate |
|---|
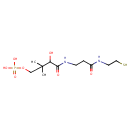
| Description | Pantetheine 4'-phosphate, also known as Pantetheine 4'-phosphate or PSH-4'-p, belongs to the class of organic compounds known as monoalkyl phosphates. These are organic compounds containing a phosphate group that is linked to exactly one alkyl chain. Pantetheine 4'-phosphate is possibly soluble (in water) and an extremely weak basic (essentially neutral) compound (based on its pKa). Pantetheine 4'-phosphate exists in all living species, ranging from bacteria to humans. Pantetheine 4'-phosphate participates in a number of enzymatic reactions, within cattle. In particular, Pantetheine 4'-phosphate can be biosynthesized from pantetheine through the action of the enzyme pantothenate kinase 1. In addition, Pantetheine 4'-phosphate can be biosynthesized from 4'-phosphopantothenoylcysteine through its interaction with the enzyme phosphopantothenoylcysteine decarboxylase. In cattle, pantetheine 4'-phosphate is involved in the metabolic pathway called pantothenate and CoA biosynthesis pathway. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 4'-Phosphopantetheine | ChEBI | | Pantotheine-4'-phosphate | ChEBI | | Phosphopantetheine | ChEBI | | PSH-4'-p | ChEBI | | Pantotheine-4'-phosphoric acid | Generator | | Pantetheine 4'-phosphoric acid | Generator | | 4'-Phosphopantotheine | HMDB | | D-Pantetheine 4'-phosphate | HMDB | | Phospho-pantotheine | HMDB | | Pantetheine-4'-phosphate | MeSH |
|
|---|
| Chemical Formula | C11H23N2O7PS |
|---|
| Average Molecular Weight | 358.348 |
|---|
| Monoisotopic Molecular Weight | 358.096358302 |
|---|
| IUPAC Name | [3-hydroxy-2,2-dimethyl-3-({2-[(2-sulfanylethyl)carbamoyl]ethyl}carbamoyl)propoxy]phosphonic acid |
|---|
| Traditional Name | pantetheine 4'-phosphate |
|---|
| CAS Registry Number | 2226-71-3 |
|---|
| SMILES | CC(C)(COP(O)(O)=O)C(O)C(=O)NCCC(=O)NCCS |
|---|
| InChI Identifier | InChI=1S/C11H23N2O7PS/c1-11(2,7-20-21(17,18)19)9(15)10(16)13-4-3-8(14)12-5-6-22/h9,15,22H,3-7H2,1-2H3,(H,12,14)(H,13,16)(H2,17,18,19) |
|---|
| InChI Key | JDMUPRLRUUMCTL-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as monoalkyl phosphates. These are organic compounds containing a phosphate group that is linked to exactly one alkyl chain. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Organic phosphoric acids and derivatives |
|---|
| Sub Class | Phosphate esters |
|---|
| Direct Parent | Monoalkyl phosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Monoalkyl phosphate
- Secondary alcohol
- Alkylthiol
- Carboximidic acid
- Carboximidic acid derivative
- Propargyl-type 1,3-dipolar organic compound
- Organic 1,3-dipolar compound
- Organic oxygen compound
- Organic nitrogen compound
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000t-7921000000-38919c105245ede94f57 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00fr-9633000000-e3c273cefb49d43405b0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-08ir-7695000000-0ee6ed099c01445060c3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01u0-9540000000-9e756a71b573d020fa6e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03nc-9300000000-d292b023d1133331f546 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-054k-9225000000-72a847152c51b3a3daa2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-9200000000-f29815698df8b18a410a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-653db2061d8b7bf264f2 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|