| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:48:23 UTC |
|---|
| Update Date | 2020-05-21 16:27:05 UTC |
|---|
| BMDB ID | BMDB0001866 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
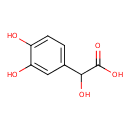
| Common Name | 3,4-Dihydroxymandelic acid |
|---|
| Description | 3,4-3,4-3,4-dihydroxymandelic acid, also known as 3,4-dihydroxymandelic acid or 3,4-dihydroxymandelic acid, belongs to the class of organic compounds known as catechols. Catechols are compounds containing a 1,2-benzenediol moiety. 3,4-3,4-3,4-dihydroxymandelic acid is possibly soluble (in water) and an extremely weak basic (essentially neutral) compound (based on its pKa). 3,4-3,4-3,4-dihydroxymandelic acid exists in all living organisms, ranging from bacteria to humans. 3,4-3,4-3,4-dihydroxymandelic acid participates in a number of enzymatic reactions, within cattle. In particular, 3,4-3,4-3,4-dihydroxymandelic acid can be biosynthesized from 3,4-dihydroxymandelaldehyde; which is mediated by the enzyme aldehyde dehydrogenase, dimeric nadp-preferring. In addition, 3,4-3,4-3,4-dihydroxymandelic acid and guaiacol can be converted into vanillylmandelic acid and pyrocatechol through the action of the enzyme catechol O-methyltransferase. In cattle, 3,4-3,4-dihydroxymandelic acid is involved in the metabolic pathway called the tyrosine metabolism pathway. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (3,4-Dihydroxyphenyl)(hydroxy)acetic acid | ChEBI | | 3,4-Dihydroxymandelate | ChEBI | | 3,4-Dihydroxyphenylglycolic acid | ChEBI | | Dihydroxymandelic acid | ChEBI | | DOMA | ChEBI | | (3,4-Dihydroxyphenyl)(hydroxy)acetate | Generator | | 3,4-Dihydroxyphenylglycolate | Generator | | Dihydroxymandelate | Generator | | 3,4 Dihydroxymandelate | HMDB | | 3,4 Dihydroxymandelic acid | HMDB | | 3,4-Dihydroxymandelic acid, (S)-isomer | HMDB | | 3,4-Dihydroxymandelic acid, ion(1-) | HMDB | | 3,4-Dihydroxymandelic acid, monosodium salt | HMDB | | DHMA | HMDB | | 3,4-Dihydroxymandelic acid, (+-)-isomer | HMDB |
|
|---|
| Chemical Formula | C8H8O5 |
|---|
| Average Molecular Weight | 184.1461 |
|---|
| Monoisotopic Molecular Weight | 184.037173366 |
|---|
| IUPAC Name | 2-(3,4-dihydroxyphenyl)-2-hydroxyacetic acid |
|---|
| Traditional Name | dihydroxymandelic acid |
|---|
| CAS Registry Number | 775-01-9 |
|---|
| SMILES | OC(C(O)=O)C1=CC=C(O)C(O)=C1 |
|---|
| InChI Identifier | InChI=1S/C8H8O5/c9-5-2-1-4(3-6(5)10)7(11)8(12)13/h1-3,7,9-11H,(H,12,13) |
|---|
| InChI Key | RGHMISIYKIHAJW-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as catechols. Catechols are compounds containing a 1,2-benzenediol moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Phenols |
|---|
| Sub Class | Benzenediols |
|---|
| Direct Parent | Catechols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Catechol
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Alpha-hydroxy acid
- Monocyclic benzene moiety
- Hydroxy acid
- Secondary alcohol
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Alcohol
- Organooxygen compound
- Aromatic alcohol
- Hydrocarbon derivative
- Carbonyl group
- Organic oxide
- Organic oxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (Non-derivatized) | splash10-0a4i-0629000000-ae329bc0399bdead30ee | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (4 TMS) | splash10-0a4i-1679200000-30b0a470753b8a534ffb | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-0a4i-0629000000-ae329bc0399bdead30ee | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-0a4i-1679200000-30b0a470753b8a534ffb | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-0a4i-0938000000-048f9a7dbf493cb105d0 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-06rl-2900000000-45a24bed7c390b0517a9 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-0a4i-1009100000-2a01ecfd802a6d86fff7 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-01qi-1900000000-54c118704077cc354da7 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-03k9-5900000000-ef520e550b3f5cce89d4 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-0a4l-7900000000-2c2914429bf7b73d4a33 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Negative | splash10-001i-0900000000-80a2a015caa693fb0bdb | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Negative | splash10-000i-0900000000-5d651845e3e03edc63dc | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Negative | splash10-000i-0900000000-5ed666462a4d020d9bad | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Negative | splash10-000i-1900000000-f785c36e33914dd6cfa0 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 50V, Negative | splash10-052r-3900000000-15fd25c25c09bb878912 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Positive | splash10-0002-9400000000-42f34c3c13cd670ff16f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Negative | splash10-000i-0900000000-e0e14e382ffa485ebcfc | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-001i-0900000000-80a2a015caa693fb0bdb | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-000i-0900000000-717de91658e43f0217e4 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-000i-0900000000-5ed666462a4d020d9bad | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-000i-1900000000-f7a0f4c346c5c2d4669f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-052r-3900000000-15fd25c25c09bb878912 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , negative | splash10-000i-0900000000-e0e14e382ffa485ebcfc | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - , negative | splash10-00kr-0900000000-bd002360ad3147f32bdb | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-0002-9400000000-42f34c3c13cd670ff16f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0900000000-1b8bb3963db8dbdf68b7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kr-0900000000-adc00447f61ba09ff2b9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0900000000-c3215810f102f9a6bcd5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0r7i-7900000000-d9fef758451d2742cf58 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001r-0900000000-6ac7602cc5f3af701662 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0apr-0900000000-b6bce5b5e83f1dd663a7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-2900000000-dfbd957390c079614963 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, experimental) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | Not Available | View in JSpectraViewer |
|---|
|
|---|