| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:20:47 UTC |
|---|
| Update Date | 2020-05-21 16:28:46 UTC |
|---|
| BMDB ID | BMDB0006242 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
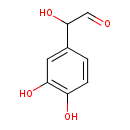
| Common Name | 3,4-Dihydroxymandelaldehyde |
|---|
| Description | 3,4-Dihydroxymandelaldehyde, also known as 3,4-dihydroxymandelaldehyde or 3,4-dihydroxymandelaldehyde, belongs to the class of organic compounds known as phenylacetaldehydes. Phenylacetaldehydes are compounds containing a phenylacetaldehyde moiety, which consists of a phenyl group substituted at the second position by an acetalydehyde. 3,4-Dihydroxymandelaldehyde is possibly soluble (in water) and an extremely weak basic (essentially neutral) compound (based on its pKa). 3,4-Dihydroxymandelaldehyde exists in all living organisms, ranging from bacteria to humans. 3,4-Dihydroxymandelaldehyde participates in a number of enzymatic reactions, within cattle. In particular, 3,4-Dihydroxymandelaldehyde can be biosynthesized from norepinephrine through the action of the enzyme amine oxidase [flavin-containing] a. In addition, 3,4-Dihydroxymandelaldehyde and methylamine can be biosynthesized from epinephrine; which is mediated by the enzyme amine oxidase [flavin-containing] a. In cattle, 3,4-dihydroxymandelaldehyde is involved in the metabolic pathway called the tyrosine metabolism pathway. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3,4-Dihydroxymandelic aldehyde | ChEBI | | 3,4-Dihydroxyphenylglycolaldehyde | ChEBI | | 3,4-Dihydroxyphenylglycolic aldehyde | ChEBI | | alpha,3,4-Trihydroxybenzeneacetaldehyde | ChEBI | | alpha,3,4-Trihydroxyphenylacetaldehyde | ChEBI | | DHMAL | ChEBI | | DHPGALD | ChEBI | | DOPEGAL | ChEBI | | a,3,4-Trihydroxybenzeneacetaldehyde | Generator | | Α,3,4-trihydroxybenzeneacetaldehyde | Generator | | a,3,4-Trihydroxyphenylacetaldehyde | Generator | | Α,3,4-trihydroxyphenylacetaldehyde | Generator | | a,3,4-Trihydroxy-benzeneacetaldehyde | HMDB | | alpha,3,4-Trihydroxy-benzeneacetaldehyde | HMDB |
|
|---|
| Chemical Formula | C8H8O4 |
|---|
| Average Molecular Weight | 168.1467 |
|---|
| Monoisotopic Molecular Weight | 168.042258744 |
|---|
| IUPAC Name | 2-(3,4-dihydroxyphenyl)-2-hydroxyacetaldehyde |
|---|
| Traditional Name | dhmal |
|---|
| CAS Registry Number | 13023-73-9 |
|---|
| SMILES | OC(C=O)C1=CC(O)=C(O)C=C1 |
|---|
| InChI Identifier | InChI=1S/C8H8O4/c9-4-8(12)5-1-2-6(10)7(11)3-5/h1-4,8,10-12H |
|---|
| InChI Key | YUGMCLJIWGEKCK-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenylacetaldehydes. Phenylacetaldehydes are compounds containing a phenylacetaldehyde moiety, which consists of a phenyl group substituted at the second position by an acetalydehyde. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Phenylacetaldehydes |
|---|
| Direct Parent | Phenylacetaldehydes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenylacetaldehyde
- Catechol
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Alpha-hydroxyaldehyde
- Secondary alcohol
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Organooxygen compound
- Organic oxygen compound
- Carbonyl group
- Aldehyde
- Aromatic alcohol
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-06ri-1900000000-d14339278a251a7d9df1 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-01b9-7079000000-441c5f66bee2b8f14be7 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0900000000-01c8f9ca75c86beb7118 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0gb9-0900000000-30165da526b8042721a9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0kn9-9800000000-24ef8505a6b8f892128d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0900000000-2ce2cd9a3f1a5d4737b3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0aor-0900000000-a9b6cf257bb2c852ffd9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-6900000000-1f2d65b1c1a32c4ea1fd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014j-0900000000-876434025a3e5951556f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0900000000-667ec91845715350734f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9200000000-1663e47bc16a5fa465e4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0fki-0900000000-d67f635e503103dc887e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0r7i-4900000000-0f535b7035498a1a6303 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ue9-9200000000-344fc9120de4f34191d3 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|