| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:44:38 UTC |
|---|
| Update Date | 2020-05-21 16:28:32 UTC |
|---|
| BMDB ID | BMDB0001354 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
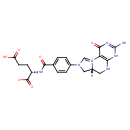
| Common Name | 5,10-Methenyltetrahydrofolic acid |
|---|
| Description | 5,10-5,10-5,10-methenyltetrahydrofolic acid, also known as 5,10-5,10-methenyltetrahydrofolic acid or 5,10-methenyl-THF, belongs to the class of organic compounds known as tetrahydrofolic acids. These are heterocyclic compounds based on the 5,6,7,8-tetrahydropteroic acid skeleton conjugated with at least one L-glutamic acid unit. 5,10-5,10-5,10-methenyltetrahydrofolic acid is possibly soluble (in water) and a moderately basic compound (based on its pKa). 5,10-5,10-5,10-methenyltetrahydrofolic acid exists in all living species, ranging from bacteria to humans. 5,10-5,10-5,10-methenyltetrahydrofolic acid participates in a number of enzymatic reactions, within cattle. In particular, 5,10-5,10-5,10-methenyltetrahydrofolic acid can be biosynthesized from 10-formyltetrahydrofolate; which is catalyzed by the enzyme C-1-tetrahydrofolate synthase, cytoplasmic. In addition, 5,10-5,10-5,10-methenyltetrahydrofolic acid can be biosynthesized from N5-formyl-THF; which is mediated by the enzyme 5-formyltetrahydrofolate cyclo-ligase. In cattle, 5,10-5,10-methenyltetrahydrofolic acid is involved in the metabolic pathway called the folate metabolism pathway. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 5,10-Methenyltetrahydrofolate | Generator | | 5,10-Methenyl-THF | HMDB | | Anhydro-leucovorin | HMDB | | Anhydro-leucovorin a | HMDB | | Anhydroleucovorin | HMDB | | Anhydroleucovorin a | HMDB | | CH-THF | HMDB | | Methenyl-tetrahydrofolate | HMDB | | Methenyl-THF | HMDB | | Methenyltetrahydrofolate | HMDB | | Methenyltetrahydrofolic acid | HMDB | | N5-N10-CH-THF | HMDB | | N5-N10-Methenyltetrahydrofolate | HMDB | | 5,10-Methenyltetrahydropteroylglutamate | HMDB | | N5,N10-Methenyl tetrahydrofolate | HMDB |
|
|---|
| Chemical Formula | C20H21N7O6 |
|---|
| Average Molecular Weight | 455.424 |
|---|
| Monoisotopic Molecular Weight | 455.155331439 |
|---|
| IUPAC Name | (6aR)-8-(4-{[(1S)-1,3-dicarboxypropyl]-C-hydroxycarbonimidoyl}phenyl)-1-hydroxy-3-imino-3H,4H,5H,6H,6aH,7H-8lambda5-imidazo[1,5-f]pteridin-8-ylium |
|---|
| Traditional Name | (6aR)-8-(4-{[(1S)-1,3-dicarboxypropyl]-C-hydroxycarbonimidoyl}phenyl)-1-hydroxy-3-imino-4H,5H,6H,6aH,7H-8lambda5-imidazo[1,5-f]pteridin-8-ylium |
|---|
| CAS Registry Number | 7444-29-3 |
|---|
| SMILES | [H][C@@]12CN(C=[N+]1C1=C(NC2)NC(N)=NC1=O)C1=CC=C(C=C1)C(=O)N[C@@H](CCC(O)=O)C([O-])=O |
|---|
| InChI Identifier | InChI=1S/C20H21N7O6/c21-20-24-16-15(18(31)25-20)27-9-26(8-12(27)7-22-16)11-3-1-10(2-4-11)17(30)23-13(19(32)33)5-6-14(28)29/h1-4,9,12-13H,5-8H2,(H6-,21,22,23,24,25,28,29,30,31,32,33)/t12-,13+/m1/s1 |
|---|
| InChI Key | MEANFMOQMXYMCT-OLZOCXBDSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as tetrahydrofolic acids. These are heterocyclic compounds based on the 5,6,7,8-tetrahydropteroic acid skeleton conjugated with at least one L-glutamic acid unit. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pteridines and derivatives |
|---|
| Sub Class | Pterins and derivatives |
|---|
| Direct Parent | Tetrahydrofolic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tetrahydrofolic acid
- Glutamic acid or derivatives
- N-acyl-alpha amino acid or derivatives
- N-acyl-alpha-amino acid
- Hippuric acid
- Hippuric acid or derivatives
- Alpha-amino acid or derivatives
- Benzamide
- Benzoic acid or derivatives
- Aniline or substituted anilines
- Benzoyl
- Aminopyrimidine
- Pyrimidone
- Secondary aliphatic/aromatic amine
- Monocyclic benzene moiety
- Pyrimidine
- Dicarboxylic acid or derivatives
- Benzenoid
- Vinylogous amide
- Heteroaromatic compound
- 2-imidazoline
- Carboxamide group
- Amino acid
- Secondary carboxylic acid amide
- Carboxylic acid salt
- Amino acid or derivatives
- Amidine
- Azacycle
- Carboxylic acid amidine
- Organic 1,3-dipolar compound
- Carboxylic acid derivative
- Propargyl-type 1,3-dipolar organic compound
- Carboxylic acid
- Secondary amine
- Organic oxygen compound
- Amine
- Carbonyl group
- Organic salt
- Organic nitrogen compound
- Organopnictogen compound
- Organic oxide
- Organic zwitterion
- Hydrocarbon derivative
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-03di-2408900000-90c26f197ed01948843b | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0230-9506630000-d9b025a3f36832b17d97 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0000900000-9a74d30be444557240b0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0000900000-87ffec278a0d552070d3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-056r-9210100000-8e44a81962b4227dbc32 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0000900000-6e21d81553cf048dcee3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-2000900000-e3e2170131566f2c34e9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9300100000-8e0fa01b9b99e8b1fea8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0003900000-a08e1d1cb1452492cc40 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0bt9-2109100000-a099f262b4e99916ab71 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-053r-0396000000-4a34a1ca0c2780791f62 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0f79-0004900000-b7d25e9a9f2b33373222 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001u-4129500000-674f21af43f4a24fc9aa | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0f9y-9643100000-a3b87dfc44080a520c03 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|