| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:45:51 UTC |
|---|
| Update Date | 2020-05-21 16:28:46 UTC |
|---|
| BMDB ID | BMDB0001430 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | L-Dopachrome |
|---|
| Description | L-Dopachrome belongs to the class of organic compounds known as l-alpha-amino acids. These are alpha amino acids which have the L-configuration of the alpha-carbon atom. Based on a literature review a small amount of articles have been published on L-Dopachrome. |
|---|
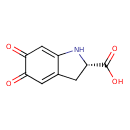
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-L-Carboxy-2,3-dihydroindole-5,6-quinone | ChEBI | | (2S)-2,3,5,6-Tetrahydro-5,6-dioxo-1H-indole-2-carboxylic acid | HMDB | | (2S)-5,6-Dioxo-2,3,5,6-tetrahydro-1H-indole-2-carboxylic acid | HMDB | | L-Dopachrome | HMDB |
|
|---|
| Chemical Formula | C9H7NO4 |
|---|
| Average Molecular Weight | 193.158 |
|---|
| Monoisotopic Molecular Weight | 193.037507709 |
|---|
| IUPAC Name | (2S)-5,6-dioxo-2,3,5,6-tetrahydro-1H-indole-2-carboxylic acid |
|---|
| Traditional Name | L-dopachrome |
|---|
| CAS Registry Number | 89762-39-0 |
|---|
| SMILES | OC(=O)[C@@H]1CC2=CC(=O)C(=O)C=C2N1 |
|---|
| InChI Identifier | InChI=1S/C9H7NO4/c11-7-2-4-1-6(9(13)14)10-5(4)3-8(7)12/h2-3,6,10H,1H2,(H,13,14)/t6-/m0/s1 |
|---|
| InChI Key | VJNCICVKUHKIIV-LURJTMIESA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as l-alpha-amino acids. These are alpha amino acids which have the L-configuration of the alpha-carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | L-alpha-amino acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - L-alpha-amino acid
- Indole or derivatives
- Dihydroindole
- Pyrrolidine carboxylic acid
- Pyrrolidine carboxylic acid or derivatives
- Pyrrolidine
- Vinylogous amide
- Amino acid
- Ketone
- Cyclic ketone
- Carboxylic acid
- Secondary aliphatic amine
- Enamine
- Monocarboxylic acid or derivatives
- Azacycle
- Secondary amine
- Organoheterocyclic compound
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organonitrogen compound
- Organooxygen compound
- Amine
- Hydrocarbon derivative
- Carbonyl group
- Organic nitrogen compound
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-002f-0900000000-7d241073b22105a8fd50 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0900000000-323f4bcd8528fb467c2b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00ba-7900000000-13c63b10c546db28b452 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0900000000-7278b78e7703333d1049 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-006w-0900000000-37fa92b16a4a5557156c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-006w-2900000000-a811e0992f3245b7b855 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0900000000-becbc589150d5f7b8591 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0900000000-96e2a94dd742e6e1937c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-006w-1900000000-1956f467b1b38dab6842 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0900000000-ae6d437a06113eb43338 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004m-0900000000-5329f1d5b80270050bbb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-006y-8900000000-bfc43ab0afc8b1b28daf | View in MoNA |
|---|
|
|---|