| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:47:31 UTC |
|---|
| Update Date | 2020-05-21 16:28:51 UTC |
|---|
| BMDB ID | BMDB0001547 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Corticosterone |
|---|
| Description | Corticosterone, also known as Corticosterone, belongs to the class of organic compounds known as 21-hydroxysteroids. These are steroids carrying a hydroxyl group at the 21-position of the steroid backbone. Thus, corticosterone is considered to be a steroid lipid molecule. Corticosterone exists as a solid, very hydrophobic, practically insoluble (in water), and relatively neutral molecule. Corticosterone participates in a number of enzymatic reactions, within cattle. In particular, Corticosterone can be converted into 18-hydroxycorticosterone through its interaction with the enzyme cytochrome P450 11B1. In addition, Corticosterone can be biosynthesized from 11b-hydroxyprogesterone; which is catalyzed by the enzyme steroid 21-hydroxylase. In cattle, corticosterone is involved in the metabolic pathway called the steroidogenesis pathway. Corticosterone is a potentially toxic compound. |
|---|
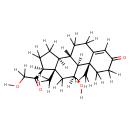
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (11beta)-11,21-Dihydroxypregn-4-ene-3,20-dione | ChEBI | | 11beta,21-Dihydroxy-4-pregnene-3,20-dione | ChEBI | | 11beta,21-Dihydroxyprogesterone | ChEBI | | 17-Deoxycortisol | ChEBI | | Kendall's compound b | ChEBI | | Reichstein's substance H | ChEBI | | (11b)-11,21-Dihydroxypregn-4-ene-3,20-dione | Generator | | (11Β)-11,21-dihydroxypregn-4-ene-3,20-dione | Generator | | 11b,21-Dihydroxy-4-pregnene-3,20-dione | Generator | | 11Β,21-dihydroxy-4-pregnene-3,20-dione | Generator | | 11b,21-Dihydroxyprogesterone | Generator | | 11Β,21-dihydroxyprogesterone | Generator | | 11,21-Dihydroxypregn-4-ene-3,20-dione | HMDB | | 11,21-Dihydroxyprogesterone | HMDB | | 11-Hydroxycorticoaldosterone | HMDB | | 4-Pregnene-11 corticosteron | HMDB |
|
|---|
| Chemical Formula | C21H30O4 |
|---|
| Average Molecular Weight | 346.4605 |
|---|
| Monoisotopic Molecular Weight | 346.214409448 |
|---|
| IUPAC Name | (1S,2R,10S,11S,14S,15S,17S)-17-hydroxy-14-(2-hydroxyacetyl)-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-en-5-one |
|---|
| Traditional Name | (1S,2R,10S,11S,14S,15S,17S)-17-hydroxy-14-(2-hydroxyacetyl)-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-en-5-one |
|---|
| CAS Registry Number | 50-22-6 |
|---|
| SMILES | [H]OC([H])([H])C(=O)[C@@]1([H])C([H])([H])C([H])([H])[C@@]2([H])[C@]3([H])C([H])([H])C([H])([H])C4=C([H])C(=O)C([H])([H])C([H])([H])[C@]4(C([H])([H])[H])[C@@]3([H])[C@@]([H])(O[H])C([H])([H])[C@]12C([H])([H])[H] |
|---|
| InChI Identifier | InChI=1S/C21H30O4/c1-20-8-7-13(23)9-12(20)3-4-14-15-5-6-16(18(25)11-22)21(15,2)10-17(24)19(14)20/h9,14-17,19,22,24H,3-8,10-11H2,1-2H3/t14-,15-,16+,17-,19+,20-,21-/m0/s1 |
|---|
| InChI Key | OMFXVFTZEKFJBZ-HJTSIMOOSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 21-hydroxysteroids. These are steroids carrying a hydroxyl group at the 21-position of the steroid backbone. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Hydroxysteroids |
|---|
| Direct Parent | 21-hydroxysteroids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Progestogin-skeleton
- 21-hydroxysteroid
- Pregnane-skeleton
- 20-oxosteroid
- 3-oxo-delta-4-steroid
- 3-oxosteroid
- 11-hydroxysteroid
- 11-beta-hydroxysteroid
- Oxosteroid
- Delta-4-steroid
- Cyclohexenone
- Alpha-hydroxy ketone
- Cyclic alcohol
- Ketone
- Secondary alcohol
- Cyclic ketone
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Organic oxygen compound
- Alcohol
- Organooxygen compound
- Primary alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Endoplasmic reticulum
- Membrane
- Mitochondria
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 179 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 0.199 mg/mL | Not Available | | LogP | 1.94 | HANSCH,C ET AL. (1995) |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (2 MEOX; 2 TMS) | splash10-0f9l-4920000000-13c56138ef34befd6786 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (2 MEOX; 2 TMS) | splash10-0f7c-4921100000-1d2ace868fd94080c88e | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-014i-1798000000-b39fe3c8aaf8f09d7cde | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-0f9l-4920000000-13c56138ef34befd6786 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-0f7c-4921100000-1d2ace868fd94080c88e | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000i-1968000000-377b49ad20a23591ce73 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-004i-2640900000-6a05b10a0e80ea76b38a | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-0002-0009000000-81d0a5e41401c962645e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-05fs-2910000000-7a585bd042169de23cfe | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-0597-6900000000-4c9bb40342921d7ff55e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - EI-B (HITACHI M-80) , Positive | splash10-014i-1798000000-e8ef26b439701fc96537 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-0002-0759000000-8c04fadb6bd7d3e86c28 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-05gi-3920000000-db0dc9413f5ca5cb69e3 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-0002-0009000000-362accabc0136898e549 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-0002-0459000000-e31660ef75e29d9d9d78 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-00fr-0941000000-1453953f7526fa9b8de6 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-00e9-0920000000-da03f39d4590a6248157 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-00gi-0900000000-2ec9e62e392a282f6de4 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-0002-0009000000-1db6d72ffc02b6efa63d | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-00ba-1964000000-d35661aaf929a1609854 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-00dj-2920000000-b29d21507e63bc379164 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-05fv-3900000000-8cd56447e4fa2da8c30b | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-0596-4900000000-0fdf5a6f27425f6540ba | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-052f-6900000000-d6cd49302b0991f7a681 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-00mo-9800000000-fcf363e8a5a69914ad88 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-00mp-9600000000-493d479336ae32a15124 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004j-0029000000-7c715165a4b71930bea5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-02dj-0349000000-ccef7282ed548096a4d8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-06dj-4391000000-c5bd1940f400fd8a11f1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0009000000-e0a478591970f0721633 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00mk-2049000000-d03aea1301697772cf2e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0abi-3092000000-80dac4c435cb4f3567a6 | View in MoNA |
|---|
| MS | Mass Spectrum (Electron Ionization) | splash10-014i-8953000000-cdd7d53a6012a6eb3f28 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, experimental) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, CDCl3, experimental) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 25.16 MHz, CDCl3, experimental) | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, CDCl3, experimental) | Not Available | View in JSpectraViewer |
|---|
|
|---|